Chemistry, 01.07.2021 16:00 ErrorNameTaken505
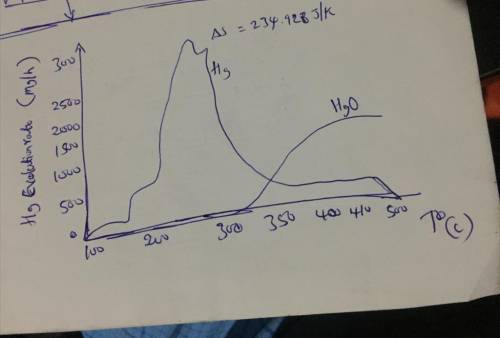
1. Show that heat flows spontaneously from high temperature to low temperature in any isolated system (hint: use entropy change that occurs during the process for your proof). 2. Work out the entropy change for the decomposition of mercuric oxide using mathematical and graphical arguments.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
1. Show that heat flows spontaneously from high temperature to low temperature in any isolated syste...
Questions

Mathematics, 14.01.2020 02:31

Social Studies, 14.01.2020 02:31

Physics, 14.01.2020 02:31


Physics, 14.01.2020 02:31

Geography, 14.01.2020 02:31


Mathematics, 14.01.2020 02:31


Geography, 14.01.2020 02:31

Biology, 14.01.2020 02:31

English, 14.01.2020 02:31

Physics, 14.01.2020 02:31

History, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31


Mathematics, 14.01.2020 02:31


Mathematics, 14.01.2020 02:31

Mathematics, 14.01.2020 02:31