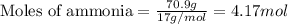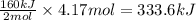2NH3(g)→N2(g)+3H2(g)

Chemistry, 01.07.2021 15:40 rowdycar313p0ao5k
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
ΔH=160kJUse the information to answer the following questions. This reaction is:.
a. endothermic
b. exothermic
Suppose 70.9 g of NH3 react. Will any heat be released or absorbed?
a. Yes, absorbed
b. Yes, released
c. No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
A chemist measures the energy change
ΔH during the following reaction:
2NH3(g)→N2(g)+3H2(g)
2NH3(g)→N2(g)+3H2(g)
Questions


Chemistry, 06.11.2020 21:30

English, 06.11.2020 21:30



Health, 06.11.2020 21:30






Chemistry, 06.11.2020 21:30


History, 06.11.2020 21:30

English, 06.11.2020 21:30


Mathematics, 06.11.2020 21:30

Mathematics, 06.11.2020 21:30


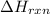 is positive for these reactions.
is positive for these reactions.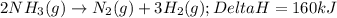
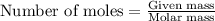 ......(1)
......(1)