
Chemistry, 30.06.2021 21:40 Attaullah8207
How many moles of lithium nitrate are theoretically produced if we start with 3.0 moles of Ca(NO3)2 and 3.0 moles of Li3PO4? Reaction: 3Ca(NO3)2 + 2Li3PO4 → 6LiNO3 + Ca3(PO4)2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
How many moles of lithium nitrate are theoretically produced if we start with 3.0 moles of Ca(NO3)2...
Questions


English, 23.02.2021 16:10



Mathematics, 23.02.2021 16:10






Mathematics, 23.02.2021 16:10



Social Studies, 23.02.2021 16:10


World Languages, 23.02.2021 16:10

Biology, 23.02.2021 16:10



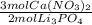 = 4.5 mol Ca(NO₃)₂
= 4.5 mol Ca(NO₃)₂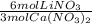 = 6.0 mol LiNO₃
= 6.0 mol LiNO₃

