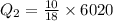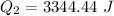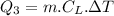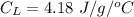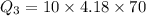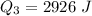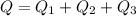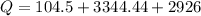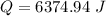Chemistry, 30.06.2021 06:50 Jerryholloway5871
The following physical constants are for water, H2O.
The specific heat capacity of the solid = 2.09 J/g oC
The specific heat capacity of the liquid = 4.18 J/g oC
The specific heat capacity of the vapor = 2.09 J/g oC
∆Hfus = 6.02 kJ/mol; ∆Hvap = 40.7 kJ/mol Freezing point = 0.0oC; Boiling point = 100.0oC
How much heat(in kJ) is required to warm 10.0 grams of ice at -5.0oC to a temperature of 70.0oC?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
The following physical constants are for water, H2O.
The specific heat capacity of the solid = 2.09...
Questions


Biology, 02.12.2021 14:00


Computers and Technology, 02.12.2021 14:00



English, 02.12.2021 14:00

Mathematics, 02.12.2021 14:00

Mathematics, 02.12.2021 14:00

World Languages, 02.12.2021 14:00


Mathematics, 02.12.2021 14:00


Spanish, 02.12.2021 14:00

Law, 02.12.2021 14:00

Biology, 02.12.2021 14:00


Arts, 02.12.2021 14:00

Biology, 02.12.2021 14:00

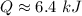
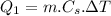
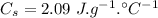
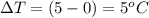
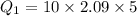
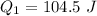
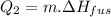
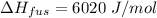
 (molecular mass)
(molecular mass)