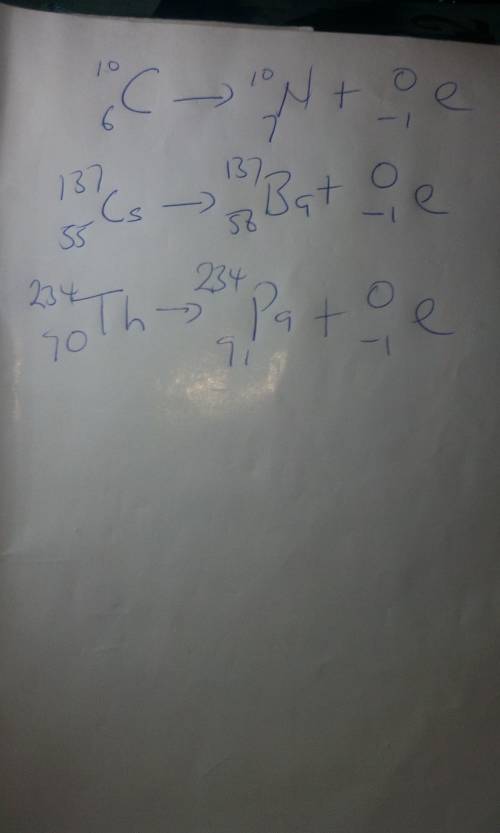Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Practice by predicting what would happen if the following underwent beta decay: (2
iv. Carbon -10 +...
Questions



Chemistry, 28.01.2021 07:50

Mathematics, 28.01.2021 07:50

Mathematics, 28.01.2021 07:50


Medicine, 28.01.2021 07:50



Mathematics, 28.01.2021 07:50

Mathematics, 28.01.2021 07:50

Mathematics, 28.01.2021 07:50

Biology, 28.01.2021 07:50

Physics, 28.01.2021 07:50

Mathematics, 28.01.2021 07:50


Health, 28.01.2021 07:50

Mathematics, 28.01.2021 07:50


Mathematics, 28.01.2021 07:50