
Chemistry, 30.06.2021 03:00 ashtonrieper1132
En la fermentación del alcohol, la levadura convierte la glucosa en etanol y dióxido de carbono:
C6H12O6(s) → 2C2H5OH(l) + 2CO2(g)
Si reaccionan 5.97 g de glucosa y se recolectan 1.44 L de CO2 gaseoso, a 293 K y 0.984 atm, ¿cuál
es el rendimiento porcentual de la reacción

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
En la fermentación del alcohol, la levadura convierte la glucosa en etanol y dióxido de carbono:
C6...
Questions



Mathematics, 24.03.2021 15:30



Mathematics, 24.03.2021 15:30

Mathematics, 24.03.2021 15:30


Mathematics, 24.03.2021 15:30


Mathematics, 24.03.2021 15:30


Mathematics, 24.03.2021 15:30

Mathematics, 24.03.2021 15:30


Mathematics, 24.03.2021 15:30

Chemistry, 24.03.2021 15:30

Mathematics, 24.03.2021 15:30


English, 24.03.2021 15:30

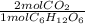 = 0.0664 mol CO₂
= 0.0664 mol CO₂


