
Chemistry, 30.06.2021 02:10 alesiabarrios6
Na3N decomposes to form sodium and nitrogen gas at STP. If 13.7 L of nitrogen is produced
how many moles of Na3N was used? (22.4 L = 1 mole of any gas)
2Na3N --> 6Na + N2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
Na3N decomposes to form sodium and nitrogen gas at STP. If 13.7 L of nitrogen is produced
how many...
Questions

Business, 04.12.2021 14:00


Arts, 04.12.2021 14:00



History, 04.12.2021 14:00

Mathematics, 04.12.2021 14:00

Mathematics, 04.12.2021 14:00



Social Studies, 04.12.2021 14:00



English, 04.12.2021 14:00



English, 04.12.2021 14:00

Mathematics, 04.12.2021 14:00

Mathematics, 04.12.2021 14:00

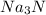 were used.
were used.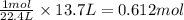
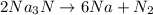
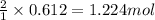 of
of 

