
Chemistry, 29.06.2021 19:00 reagancunningham2004
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl3 (g) + Cl2 (g)
A vessel is charged with PCl 5 giving an initial pressure of 0.123 atm and yields PCl 3 and Cl 2. At equilibrium, the partial pressure of PCl 3 is atm.
A) 0.0782.
B) 0.0455.
C) 0.0908.
D) 0.0330.
E) 0.123.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
The equilibrium constant (K p) for the interconversion of PCl 5 and PCl 3 is 0.0121:
PCl5 (g) → PCl...
Questions


Mathematics, 24.03.2021 19:50


Biology, 24.03.2021 19:50



English, 24.03.2021 19:50

Biology, 24.03.2021 19:50

Mathematics, 24.03.2021 19:50


Mathematics, 24.03.2021 19:50

Mathematics, 24.03.2021 19:50

Mathematics, 24.03.2021 19:50

Social Studies, 24.03.2021 19:50


Mathematics, 24.03.2021 19:50

Biology, 24.03.2021 19:50


Mathematics, 24.03.2021 19:50

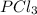 is 0.0330 atm.
is 0.0330 atm.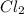 . Hence, let us assume that x quantity of
. Hence, let us assume that x quantity of 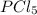 is decomposed and gives x quantity of
is decomposed and gives x quantity of 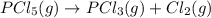
 of this reaction is as follows.
of this reaction is as follows.![K_{p} = \frac{[PCl_{3}][Cl_{2}]}{[PCl_{5}]}\\0.0121 = \frac{x \times x}{(0.123 - x)}\\x = 0.0330](/tpl/images/1386/0346/b037d.png)


