
Chemistry, 25.06.2021 14:00 maggie9459
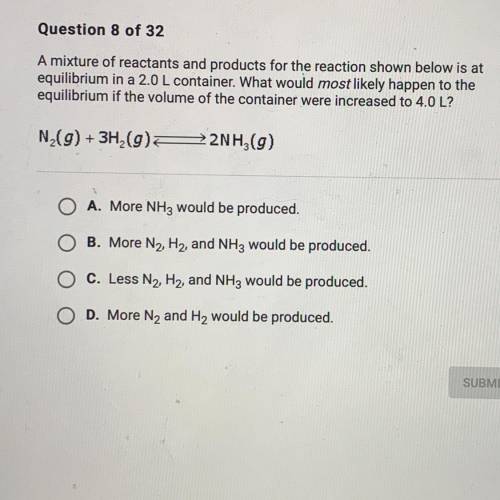
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container. What would most likely happen to the equilibrium if the volume of the container were increased to 4.0 L?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
A mixture of reactants and products for the reacting shown below is at equilibrium 2.0 L container....
Questions

Social Studies, 11.09.2021 15:00

Biology, 11.09.2021 15:00

Biology, 11.09.2021 15:00

History, 11.09.2021 15:00


History, 11.09.2021 15:00

History, 11.09.2021 15:20

Advanced Placement (AP), 11.09.2021 15:30


Chemistry, 11.09.2021 15:40

Mathematics, 11.09.2021 15:40

Mathematics, 11.09.2021 16:00

English, 11.09.2021 16:00


Mathematics, 11.09.2021 16:10

Social Studies, 11.09.2021 16:10


Social Studies, 11.09.2021 16:10


Physics, 11.09.2021 16:20



