
Chemistry, 25.06.2021 05:40 LUCASEDIE7430
C8H18+O2=CO2+H2O. Balance this equation and identify the number H2O molecules formed when 6 molecules of C8H18 react with 75 molecules of oxygen

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
C8H18+O2=CO2+H2O. Balance this equation and identify the number H2O molecules formed when 6 molecule...
Questions




Mathematics, 09.06.2020 18:57


Biology, 09.06.2020 18:57



Mathematics, 09.06.2020 18:57

Mathematics, 09.06.2020 18:57

Mathematics, 09.06.2020 18:57

Mathematics, 09.06.2020 18:57


Mathematics, 09.06.2020 18:57

Mathematics, 09.06.2020 18:57





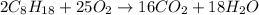
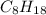 = 6
= 6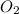 = 75
= 75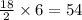 molecules of water
molecules of water

