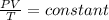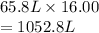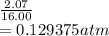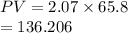Chemistry, 25.06.2021 03:10 Christyhanes3764
A flexible vessel contains 65.8 L of gas at a pressure of 2.07 atm. Under the conditions of constant temperature and constant number of moles of gas, what is the pressure of the gas (in atm) when the volume of the vessel increased by a factor of 16.00

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2
You know the right answer?
A flexible vessel contains 65.8 L of gas at a pressure of 2.07 atm. Under the conditions of constant...
Questions


Social Studies, 03.09.2020 08:01

History, 03.09.2020 08:01

Chemistry, 03.09.2020 08:01

Mathematics, 03.09.2020 08:01


English, 03.09.2020 08:01

Geography, 03.09.2020 08:01


Mathematics, 03.09.2020 08:01




Mathematics, 03.09.2020 08:01

Business, 03.09.2020 08:01



History, 03.09.2020 08:01

History, 03.09.2020 08:01

English, 03.09.2020 08:01

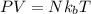
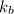 = Boltzmann constant
= Boltzmann constant