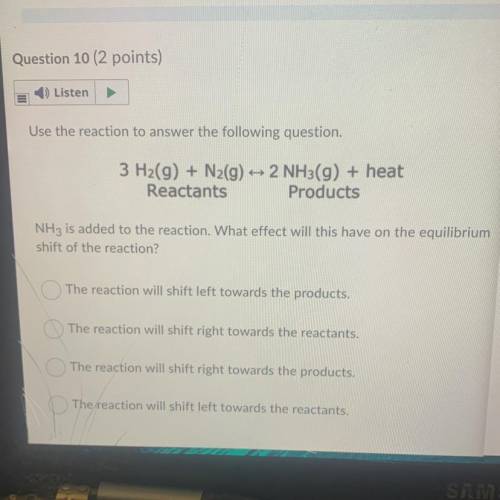
Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactant...

Use the reaction to answer the following question.
3 H2(g) + N2(g) + 2 NH3(g) + heat
Reactants Products
NH3 is added to the reaction. What effect will this have on the equilibrium
shift of the reaction?
The reaction will shift left towards the products.
The reaction will shift right towards the reactants.
The reaction will shift right towards the products.
The reaction will shift left towards the reactants.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3


Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

You know the right answer?
Questions

Mathematics, 19.08.2021 08:30




Mathematics, 19.08.2021 08:30

Mathematics, 19.08.2021 08:30

Mathematics, 19.08.2021 08:30

Mathematics, 19.08.2021 08:30




Mathematics, 19.08.2021 08:40

Mathematics, 19.08.2021 08:40

Mathematics, 19.08.2021 08:40



Mathematics, 19.08.2021 08:40


English, 19.08.2021 08:40



