
Chemistry, 24.06.2021 22:00 aryannaholmes9
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydrogen atom.
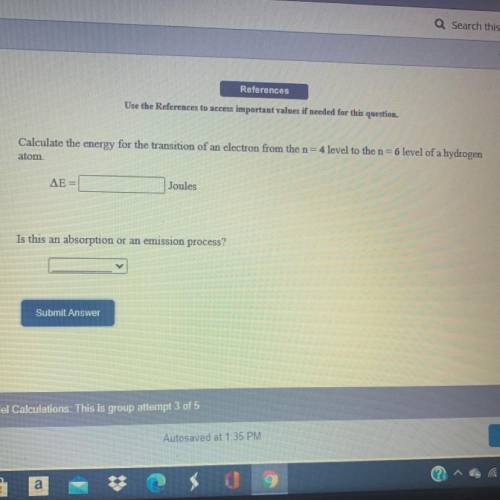

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydr...
Questions


Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00





Mathematics, 10.12.2020 01:00

English, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00


Mathematics, 10.12.2020 01:00


Social Studies, 10.12.2020 01:00

Chemistry, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Business, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

History, 10.12.2020 01:00



