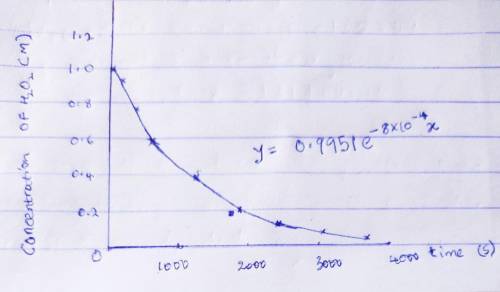
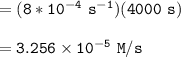The decomposition of hydrogen peroxide was studied, and the following data were obtained at a particular temperature. Time (s)[H2O2] (mol/L)0 1.00120 ± 10.91300 ± 10.78600 ± 10.591200 ± 10.371800 ± 10.222400 ± 10.133000 ± 10.0823600 ± 10.050Assuming that the rate= -delta [H2O2]/delta t determine the rate law, integrated rate law, and the value of the rate constant. Calculate [H2O2] at 4000. s after the start of the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
The decomposition of hydrogen peroxide was studied, and the following data were obtained at a partic...
Questions

Mathematics, 17.06.2021 01:50



History, 17.06.2021 01:50

Mathematics, 17.06.2021 01:50






Mathematics, 17.06.2021 02:00

Chemistry, 17.06.2021 02:00


Business, 17.06.2021 02:00

Mathematics, 17.06.2021 02:00

Mathematics, 17.06.2021 02:00

Computers and Technology, 17.06.2021 02:00



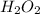 which declines exponentially in relation to the time and it obeys the equation:
which declines exponentially in relation to the time and it obeys the equation: 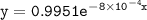
![\mathtt{ [A] = [A]_o e^{-kt}}](/tpl/images/1383/5053/858f7.png)
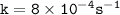
![\mathtt{= k[H_2O_2]}](/tpl/images/1383/5053/b66a7.png)
![\mathtt{[H_2O_2] = [H_2O_2]_oe^{-(8*10^{-4})t}}](/tpl/images/1383/5053/3f9ba.png)
![\mathtt{[H_2O_2] = [H_2O_2]_oe^{-8*10^{-4}(t)}}](/tpl/images/1383/5053/adf3d.png)
![\mathtt{[H_2O_2] = (1.00\ M)*e^{-8*10^{-4}(4000)\ s}}](/tpl/images/1383/5053/a221b.png)
![\mathtt{[H_2O_2] =0.0407 \ M}](/tpl/images/1383/5053/c14b9.png)