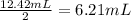Chemistry, 24.06.2021 19:10 angelequej1167
A weak acid is titrated with 0.1236 M NaOH. From the titration curve you determine that the equivalence point occurs after exactly 12.42 mL of NaOH have been added. What is the volume of NaOH at the half-equivalence point (a. k.a. the midpoint)

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
A weak acid is titrated with 0.1236 M NaOH. From the titration curve you determine that the equivale...
Questions



English, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00

English, 17.10.2021 14:00


Mathematics, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00


Physics, 17.10.2021 14:00

Spanish, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00

English, 17.10.2021 14:00

English, 17.10.2021 14:00

Physics, 17.10.2021 14:00


Mathematics, 17.10.2021 14:00

Biology, 17.10.2021 14:00

World Languages, 17.10.2021 14:00