
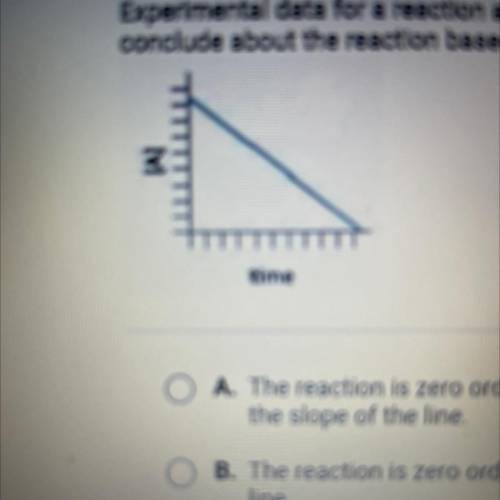
Experimental data for a reaction are collected and graphed. What can you
conclude about the reaction based on the graph?
A. The reaction is zero order, and the rate constant is the negative of
the slope of the line
B. The reaction is zero order, and the rate constant is the slope of the
line
C. The reaction is first order, and the rate constant is the slope of the
line,
D. The reaction is first order, and the rate constant is the negative eg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Experimental data for a reaction are collected and graphed. What can you
conclude about the reactio...
Questions


Computers and Technology, 22.05.2021 01:40




Mathematics, 22.05.2021 01:40

Spanish, 22.05.2021 01:40



Biology, 22.05.2021 01:40


Chemistry, 22.05.2021 01:40


Mathematics, 22.05.2021 01:40

History, 22.05.2021 01:40



English, 22.05.2021 01:40


Mathematics, 22.05.2021 01:40



