Question 22 (Essay Worth 8 points)
(05.07 HC)
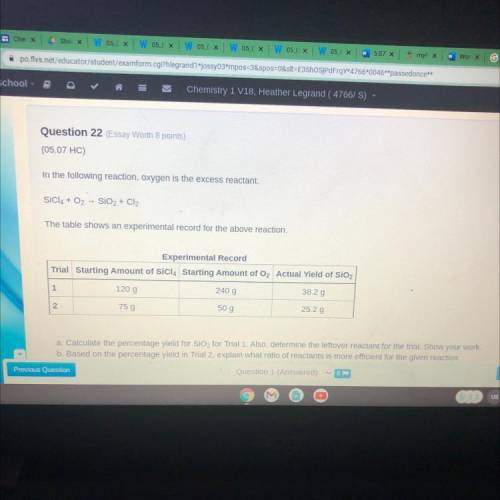
In the following reaction, oxygen is the excess...

Question 22 (Essay Worth 8 points)
(05.07 HC)
In the following reaction, oxygen is the excess reactant.
SÍCIA + O2 - SiO2 + Cl2
The table shows an experimental record for the above reaction.
Experimental Record
Trial Starting Amount of SiCl4 Starting Amount of O2 Actual Yield of SiO2
1
120 g
240 g
38.2 g
N
75 g
50 g
25.2 g
a. Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work
b. Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

You know the right answer?
Questions


English, 28.03.2020 02:43

Chemistry, 28.03.2020 02:43



Mathematics, 28.03.2020 02:43


Mathematics, 28.03.2020 02:43

Mathematics, 28.03.2020 02:43

Spanish, 28.03.2020 02:43


History, 28.03.2020 02:43





Mathematics, 28.03.2020 02:43

Mathematics, 28.03.2020 02:43

Physics, 28.03.2020 02:43



