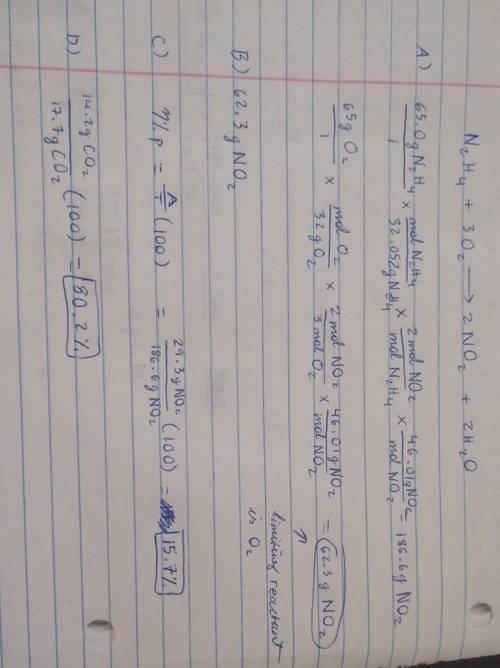19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O2(g) → 2 NO2(g) + 2 H20 (8)
A) If 65.0 g of hydrazine are reacted with 65.0 g of oxygen, which is the limiting reactant?
usg of Oz X Imol
.
B) How many grams of NO2 are produced from the limiting reactant in part A?
C) If 29.3 g of NO2 are obtained from the reaction in Part A, what is the percent yield?
20) What is the percent yield if 14.2 g of CO2 were produced and 17.7 g were calculated to be
produced from the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
19) Hydrazine, N2H4, a substance used as rocket fuel, reacts with oxygen as follows:
N2H4 (1) + 3 O...
Questions


Mathematics, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57


Mathematics, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57


Mathematics, 13.06.2020 23:57


Mathematics, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57






Social Studies, 13.06.2020 23:57

Mathematics, 13.06.2020 23:57


Mathematics, 13.06.2020 23:57