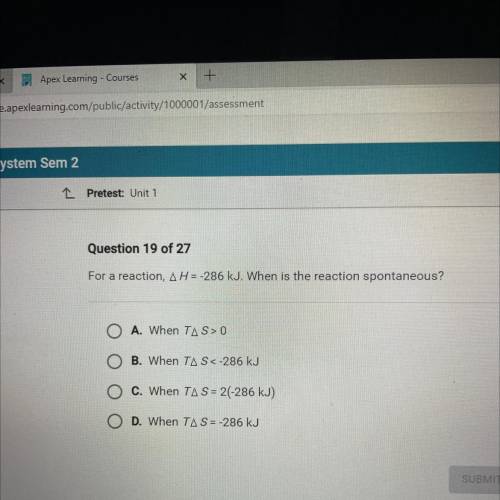
For a reaction, AH = -286 kJ. When is the reaction spontaneous?
O A. When TAS> 0
O B. When...

Chemistry, 22.06.2021 14:50 lebron06james
For a reaction, AH = -286 kJ. When is the reaction spontaneous?
O A. When TAS> 0
O B. When TAS<-286 kJ
O C. When TA S = 2(-286 kJ)
O D. When TAS = -286 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Questions

Mathematics, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00


English, 17.12.2020 01:00



Computers and Technology, 17.12.2020 01:00



Mathematics, 17.12.2020 01:00


Mathematics, 17.12.2020 01:00


English, 17.12.2020 01:00








