
Chemistry, 22.06.2021 07:10 winterchadrick
What is the quantity of
heat required to raise the
temperature of 500 g of
iron by 2°C?
The specific heat capacity
of iron is 500 J/(kg °C)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
What is the quantity of
heat required to raise the
temperature of 500 g of
iron by 2°C?...
temperature of 500 g of
iron by 2°C?...
Questions



Chemistry, 23.04.2021 21:00

Mathematics, 23.04.2021 21:00

Mathematics, 23.04.2021 21:00

History, 23.04.2021 21:00


Engineering, 23.04.2021 21:00

Chemistry, 23.04.2021 21:00

Mathematics, 23.04.2021 21:00

English, 23.04.2021 21:00


Business, 23.04.2021 21:00


Mathematics, 23.04.2021 21:00


Chemistry, 23.04.2021 21:00



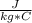 m= 500 g= 0.500 kgΔT= 2 C
m= 500 g= 0.500 kgΔT= 2 C

