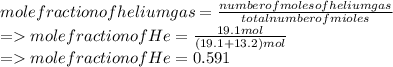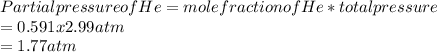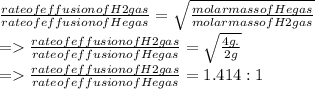Chemistry, 22.06.2021 06:00 pinkycupcakes3oxbqhx
A mixture of gases at 2.99 atm can You have two gases, and , at the same temperature. Determine the ratio of effusion rates of and .ists of 13.2 moles of hydrogen gas and 19.1 moles of helium gas. Determine the partial pressure of the helium gas.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1


Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
A mixture of gases at 2.99 atm can You have two gases, and , at the same temperature. Determine the...
Questions

Mathematics, 01.06.2020 00:58

History, 01.06.2020 00:58

Mathematics, 01.06.2020 00:58

Mathematics, 01.06.2020 00:58


History, 01.06.2020 00:58

History, 01.06.2020 00:58




Chemistry, 01.06.2020 01:57


Mathematics, 01.06.2020 01:57


Mathematics, 01.06.2020 01:57

History, 01.06.2020 01:57


Mathematics, 01.06.2020 01:57

Mathematics, 01.06.2020 01:57