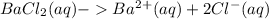Chemistry, 21.06.2021 16:30 laykaleb086
The student is now told that the four solids, in no particular order, are barium chloride (BaCl2), sugar (C6H12O6), butanoic acid (C3H7COOH), and sodium bromide (NaBr). Assuming that conductivity is correlated to the number of ions in solution, rank the four substances based on how well a 0.20 M solution in water will conduct electricity. Rank from most conductive to least conductive.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
The student is now told that the four solids, in no particular order, are barium chloride (BaCl2), s...
Questions


Mathematics, 03.02.2021 18:00

Biology, 03.02.2021 18:00

Mathematics, 03.02.2021 18:00

Biology, 03.02.2021 18:00


Health, 03.02.2021 18:00



Arts, 03.02.2021 18:00

English, 03.02.2021 18:00

Mathematics, 03.02.2021 18:00

Mathematics, 03.02.2021 18:00


Mathematics, 03.02.2021 18:00

Chemistry, 03.02.2021 18:00


Mathematics, 03.02.2021 18:00

Mathematics, 03.02.2021 18:00

Mathematics, 03.02.2021 18:00