
Chemistry, 20.06.2021 08:30 erinleyanne
Which statement is true to explain how the system below would respond to being heated.
a)Captionless Image
b)the equilibrium will shift right toward the products.
c)the forward rate would increase more than the reverse rate
d)the reverse rate would increase more than the forward rate
e)[SO₃] will increase
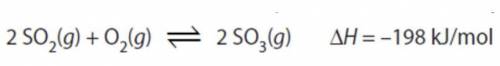

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

You know the right answer?
Which statement is true to explain how the system below would respond to being heated.
a)Captionles...
Questions

English, 16.07.2020 02:01

English, 16.07.2020 02:01

Mathematics, 16.07.2020 02:01

Mathematics, 16.07.2020 02:01


Mathematics, 16.07.2020 02:01

Spanish, 16.07.2020 02:01

Mathematics, 16.07.2020 02:01

Mathematics, 16.07.2020 02:01

Mathematics, 16.07.2020 02:01


Mathematics, 16.07.2020 02:01

Arts, 16.07.2020 02:01


Biology, 16.07.2020 02:01







