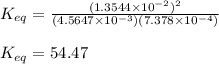PLS I REALLY NEED HELP DUE FODAY
For the reaction of hydrogen gas with iodine gas to make hydrogen iodide gas, H2 + 12 → 2HI, you have the following concentrations at equilibrium: [HQ] = 4.5647 x 10-3 M, [12] = 7.378 x 10-4 [HI] = 1.3544 x 10-2 M. M, and What is the equilibrium constant?
0.4997
0.54
46.33
54.47

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3
You know the right answer?
PLS I REALLY NEED HELP DUE FODAY
For the reaction of hydrogen gas with iodine gas to make hydrogen...
Questions

Mathematics, 07.11.2019 08:31


Mathematics, 07.11.2019 08:31


Mathematics, 07.11.2019 08:31



Mathematics, 07.11.2019 08:31

Mathematics, 07.11.2019 08:31



Social Studies, 07.11.2019 08:31




English, 07.11.2019 08:31

Mathematics, 07.11.2019 08:31


Mathematics, 07.11.2019 08:31

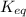
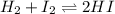
![K_{eq}=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/1379/5304/ad3b5.png)
![[HI]_{eq}=1.3544\times 10^{-2}M](/tpl/images/1379/5304/f06d4.png)
![[H_2]_{eq}=4.5647\times 10^{-3}M](/tpl/images/1379/5304/6aa27.png)
![[I_2]_{eq}=7.378\times 10^{-4}M](/tpl/images/1379/5304/297eb.png)