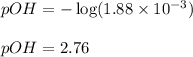Chemistry, 19.06.2021 14:00 pinkycupcakes3oxbqhx
Calculate the pH of a 0.2 M * 4 solution for which Kb = 1.8*10^-5 at 26 c . The equation for the reaction Nh3+H2O->NH4+oh

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2


Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Calculate the pH of a 0.2 M * 4 solution for which Kb = 1.8*10^-5 at 26 c . The equation for the rea...
Questions




Biology, 01.12.2021 21:50

Mathematics, 01.12.2021 21:50

History, 01.12.2021 21:50

Mathematics, 01.12.2021 21:50

Mathematics, 01.12.2021 21:50

Mathematics, 01.12.2021 21:50

English, 01.12.2021 21:50


Business, 01.12.2021 21:50




Physics, 01.12.2021 21:50


Social Studies, 01.12.2021 21:50


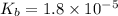
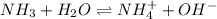
![K_b=\frac{[NH_4^+][OH^-]}{[NH_3]}](/tpl/images/1379/3913/00f50.png)
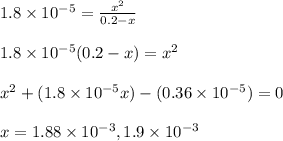
![[OH^-]=x=1.88\times 10^{-3}M](/tpl/images/1379/3913/b0c22.png)
![pOH=-\log [OH^-]](/tpl/images/1379/3913/1fac1.png)