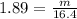Chemistry, 19.06.2021 05:30 thegreentnt5025
A 16.4L sample of NO2(S) has a density of 1.89 g/L. What is the mass of the sample NO2(s)?
A) 31.0 grams
B) 14.5 grams
C) 8.68 grams
D) 0.115 grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
A 16.4L sample of NO2(S) has a density of 1.89 g/L. What is the mass of the sample NO2(s)?
A) 31.0...
Questions


History, 27.10.2020 22:20

Biology, 27.10.2020 22:20


English, 27.10.2020 22:20

Biology, 27.10.2020 22:20



Mathematics, 27.10.2020 22:20

Chemistry, 27.10.2020 22:20

History, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20



English, 27.10.2020 22:20

Mathematics, 27.10.2020 22:20

Biology, 27.10.2020 22:20



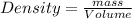 , or
, or 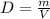 .
.