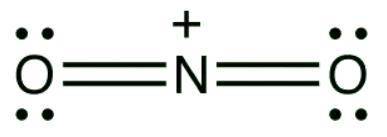A mixture of sulfuric acid and nitric acid will produce small quantities of the nitronium ion (NO2 ): The nitronium ion has a central nitrogen atom with a positive charge double bonded to two oxygen atoms on both sides. Each oxygen atom carries two lone pairs of electrons. Does the nitronium ion have any significant resonance structures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
A mixture of sulfuric acid and nitric acid will produce small quantities of the nitronium ion (NO2 )...
Questions



Mathematics, 28.11.2019 21:31















Chemistry, 28.11.2019 21:31


Physics, 28.11.2019 21:31