
Chemistry, 18.06.2021 20:00 kassandrarosario1115
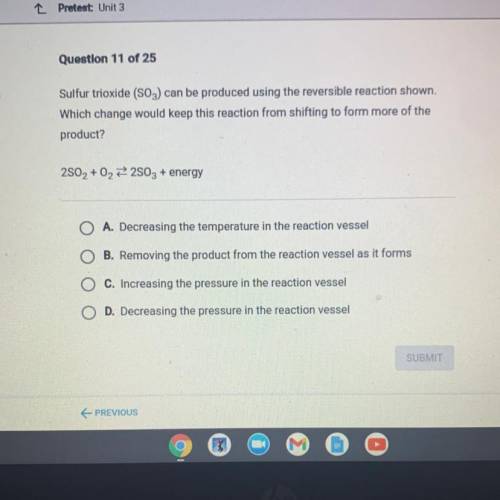
Sulfur trioxide (SO3) can be produced using the reversible reaction shown.
Which change would keep this reaction from shifting to form more of the
product?
2802 + 02 22803 + energy
A. Decreasing the temperature in the reaction vessel
B. Removing the product from the reaction vessel as it forms
C. Increasing the pressure in the reaction vessel
D. Decreasing the pressure in the reaction vessel

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Sulfur trioxide (SO3) can be produced using the reversible reaction shown.
Which change would keep...
Questions

Mathematics, 24.04.2020 23:35


Mathematics, 24.04.2020 23:35

Mathematics, 24.04.2020 23:35

English, 24.04.2020 23:35

Computers and Technology, 24.04.2020 23:35











English, 24.04.2020 23:35


Mathematics, 24.04.2020 23:35



