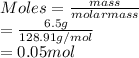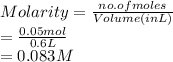Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3


Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
Vincent dissolves 6.5 g of bromic acid, HBrO3, to prepare 600.0 mL of solution. Calculate the concen...
Questions

Computers and Technology, 25.02.2021 05:30

Chemistry, 25.02.2021 05:30



English, 25.02.2021 05:40




Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40






Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40