Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2
You know the right answer?
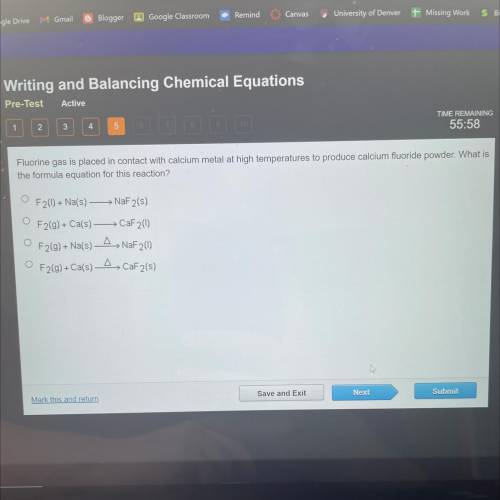
Fluorine gas is placed in contact with calcium metal at high temperatures to produce calcium fluorid...
Questions






Social Studies, 06.10.2019 17:10


Chemistry, 06.10.2019 17:10

Social Studies, 06.10.2019 17:10

Biology, 06.10.2019 17:10





Biology, 06.10.2019 17:10

History, 06.10.2019 17:10

Spanish, 06.10.2019 17:10

English, 06.10.2019 17:10