
Chemistry, 17.06.2021 05:00 shashi2728
The reaction (image below) can be abbreviated as HCl → H+ + Cl-. How is this apparently important omission justified?
A)
It isn't justified, and the abbreviation is an error.
B)
It's understood that H+ doesn't exist on its own in water, and image are both aqueous.
C)
It's generally understood that H3O+ is a hypothetical intermediary.
D)
Because water isn't important to the reaction
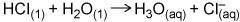

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
The reaction (image below) can be abbreviated as HCl → H+ + Cl-. How is this apparently important om...
Questions



Mathematics, 06.11.2020 16:50



Mathematics, 06.11.2020 16:50



Mathematics, 06.11.2020 16:50









Mathematics, 06.11.2020 16:50




