
Chemistry, 17.06.2021 05:00 tayler6289
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems. (HELP ASAAAP)
A)
1.68 V
B)
0.78 V
C)
–1.68 V
D)
–0.78 V
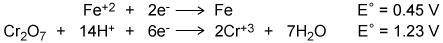

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell p...
Questions


Biology, 09.09.2019 20:30


Geography, 09.09.2019 20:30

English, 09.09.2019 20:30





Geography, 09.09.2019 20:30


History, 09.09.2019 20:30

Physics, 09.09.2019 20:30


Mathematics, 09.09.2019 20:30



Physics, 09.09.2019 20:30

Health, 09.09.2019 20:30



