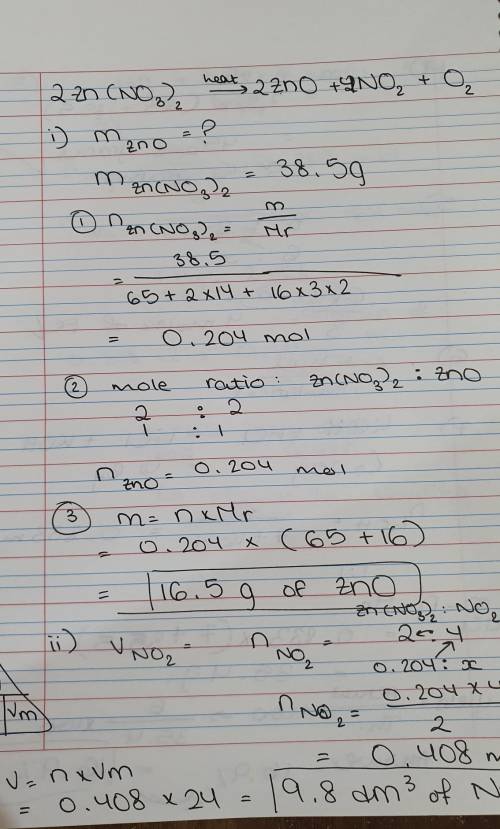When zinc nitrate is heated, zinc oxide, nitrogen dioxide(NO2) and oxygen gas are
produced.
i...

Chemistry, 16.06.2021 16:00 megankbrown
When zinc nitrate is heated, zinc oxide, nitrogen dioxide(NO2) and oxygen gas are
produced.
i.
Calculate the mass of Zinc oxide produced if 38.5 g of zinc nitrate is heated.
ii.
Determine the volume of Nitrogen dioxide gas evolved at rtp

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
Questions


Mathematics, 03.10.2019 08:30

History, 03.10.2019 08:30

Mathematics, 03.10.2019 08:30


Mathematics, 03.10.2019 08:30

History, 03.10.2019 08:30

Mathematics, 03.10.2019 08:30

Mathematics, 03.10.2019 08:30

Mathematics, 03.10.2019 08:30

English, 03.10.2019 08:30

Social Studies, 03.10.2019 08:30




Mathematics, 03.10.2019 08:30

Biology, 03.10.2019 08:30

Business, 03.10.2019 08:30