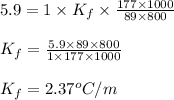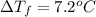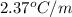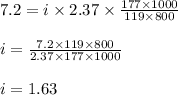Chemistry, 16.06.2021 15:40 PersonPerson13260
When of alanine are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . Calculate the mass of potassium bromide that must be dissolved in the same mass of to produce the same depression in freezing point. The van't Hoff factor for potassium bromide in .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
When of alanine are dissolved in of a certain mystery liquid , the freezing point of the solution is...
Questions


History, 30.09.2020 04:01



Mathematics, 30.09.2020 04:01



History, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01


English, 30.09.2020 04:01

Mathematics, 30.09.2020 04:01

Advanced Placement (AP), 30.09.2020 04:01

Mathematics, 30.09.2020 04:01



Chemistry, 30.09.2020 04:01

Biology, 30.09.2020 04:01


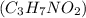 are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is
are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is 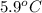 lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is
lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is 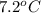 lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.
lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.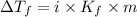
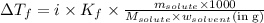 ......(1)
......(1)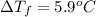
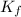 = freezing point depression constant
= freezing point depression constant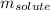 = Given mass of solute (alanine) = 177. g
= Given mass of solute (alanine) = 177. g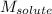 = Molar mass of solute (alanine) = 89 g/mol
= Molar mass of solute (alanine) = 89 g/mol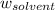 = Mass of solvent = 800.0 g
= Mass of solvent = 800.0 g