
Chemistry, 11.10.2019 19:30 rowdycar313p0ao5k
What is the theoretical yield of ammonia that can be obtained from the reaction of 10.0 g of h2 and excess n2?
n2 + 3h2 → 2nh3
a. 90.0 g
b. 97.1 g
c. 56.3 g
d. 48.6 g
e. 28.4 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
What is the theoretical yield of ammonia that can be obtained from the reaction of 10.0 g of h2 and...
Questions











Mathematics, 09.12.2019 21:31



History, 09.12.2019 21:31




Mathematics, 09.12.2019 21:31

Mathematics, 09.12.2019 21:31

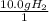 ×
×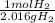 ×
×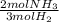 ×
×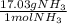 =56.3gNH↓3
=56.3gNH↓3

