
Chemistry, 16.06.2021 07:30 babygirl123468
An inflated balloon contains X air molecules. After some time the volume of the balloon is found to be the half at the same temperature and pressure when a few air molecules are expelled out. a)How many molecules will be there in the balloon now? b) Which is the gas law associated with this?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
An inflated balloon contains X air molecules. After some time the volume of the balloon is found to...
Questions

Computers and Technology, 07.12.2020 18:40

Biology, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40








Mathematics, 07.12.2020 18:40

Chemistry, 07.12.2020 18:40


Mathematics, 07.12.2020 18:40

Chemistry, 07.12.2020 18:40


Biology, 07.12.2020 18:40

Mathematics, 07.12.2020 18:40


Mathematics, 07.12.2020 18:40

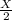 number of molecules now.
number of molecules now.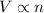 (At constant pressure and temperature)
(At constant pressure and temperature)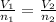 .....(1)
.....(1) = Initial volume and number of moles
= Initial volume and number of moles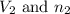 = Final volume and number of moles
= Final volume and number of moles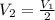
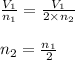
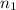 number of moles of gas contains X number of molecules
number of moles of gas contains X number of molecules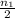 number of moles of gas will contain =
number of moles of gas will contain = 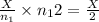 number of molecules
number of molecules

