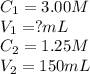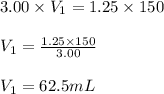Chemistry, 15.06.2021 20:30 lanettejohnson355
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solution is 150 mL. What volume of a 1.25 M solution could be made from the stock solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 18:20
Asalt is best described as a compound that is formed from the reaction between an acid and a base. a strong acid and a weak acid. a strong base and a weak base. an acid and water save and exit next submit
Answers: 1

Chemistry, 23.06.2019 21:00
Which is greater in positive acceleration, initial or final velocity
Answers: 1
You know the right answer?
A stock solution of calcium sulfate, CaSO4 has a concentration of 3.00 M. The volume of this solutio...
Questions


Mathematics, 29.10.2020 17:10



Mathematics, 29.10.2020 17:10







English, 29.10.2020 17:10


English, 29.10.2020 17:10

History, 29.10.2020 17:10




Mathematics, 29.10.2020 17:10

Biology, 29.10.2020 17:10

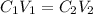 ....(1)
....(1)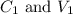 are the concentration and volume of stock solution.
are the concentration and volume of stock solution.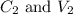 are the concentration and volume of diluted solution.
are the concentration and volume of diluted solution.