
Chemistry, 15.06.2021 19:10 nuggetslices
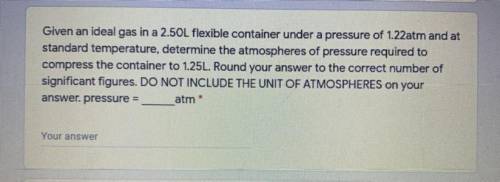
Given an ideal gas in a 2.50L flexible container under a pressure of 1.22atm and at
standard temperature, determine the atmospheres of pressure required to compress the container to 1.25L. Round your answer to the correct number of
significant figures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

You know the right answer?
Given an ideal gas in a 2.50L flexible container under a pressure of 1.22atm and at
standard temper...
Questions




Social Studies, 13.07.2019 09:20

Mathematics, 13.07.2019 09:20



Mathematics, 13.07.2019 09:20

English, 13.07.2019 09:20





Mathematics, 13.07.2019 09:20

Biology, 13.07.2019 09:20

Social Studies, 13.07.2019 09:20


Mathematics, 13.07.2019 09:20


Social Studies, 13.07.2019 09:20



