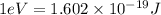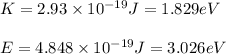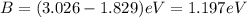(A) Calculate the wavelength (in nm) of light with energy 1.89 × 10–20 J per photon, (b) For light of wavelength 410 nm, calculate the number of photons per joule, (c) Determine the binding energy (in eV) of a metal if the kinetic energy possessed by an ejected electron [using one of the photons in part (b)] is 2.93 × 10–19 J.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1


You know the right answer?
(A) Calculate the wavelength (in nm) of light with energy 1.89 × 10–20 J per photon, (b) For light o...
Questions

History, 27.08.2019 17:30

Mathematics, 27.08.2019 17:30



Mathematics, 27.08.2019 17:30

Chemistry, 27.08.2019 17:30

Mathematics, 27.08.2019 17:30


Biology, 27.08.2019 17:30



Mathematics, 27.08.2019 17:30


Biology, 27.08.2019 17:30

Biology, 27.08.2019 17:30

Mathematics, 27.08.2019 17:30




Mathematics, 27.08.2019 17:30

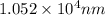
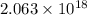
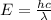 ......(1)
......(1)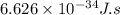
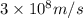
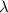 = wavelength
= wavelength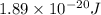
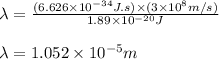
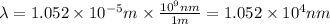
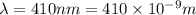
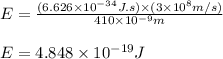
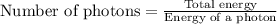
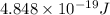
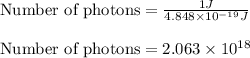
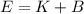 .....(2)
.....(2)