PCl5 <-> PCl3 + Cl2
PCl5 decomposes into PCl3 and Cl2 according to the equation above. A pure sample of Pcl5 is placed in a rigid, evacuated 1.00 L container. The initial pressure of the PCl5 is 1.00 atm. The temperature is held constant until the PCl5 reaches equilibrium with its decomposition products. The figures below shows the initial and equilibrium conditions of the system.
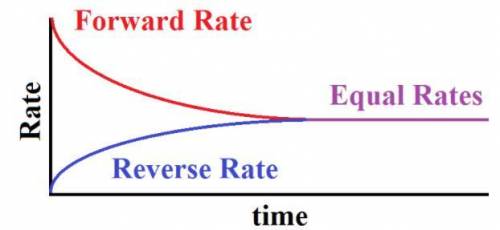
As the reaction progresses toward equilibrium, the rate of the forward reaction
A) increases until it becomes the same as the reverse reaction rate at equilibrium
B) stays constant before and after equilibrium is reached
C) decreases to become a constant nonzero rate at equilibrium
D) decreases to become zero at equilibrium

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
PCl5 <-> PCl3 + Cl2
PCl5 decomposes into PCl3 and Cl2 according to the equation above. A pure...
Questions


History, 16.09.2019 04:30



History, 16.09.2019 04:30


Mathematics, 16.09.2019 04:30


History, 16.09.2019 04:30


Biology, 16.09.2019 04:30


Biology, 16.09.2019 04:30




History, 16.09.2019 04:30


Mathematics, 16.09.2019 04:30

Social Studies, 16.09.2019 04:30