

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
You know the right answer?
Which of the following molecules would you expect to have a dipole moment of zero? a, CH2 Ch3
bH2C=...
Questions

Mathematics, 20.10.2019 20:00

Biology, 20.10.2019 20:00

Social Studies, 20.10.2019 20:00

Health, 20.10.2019 20:00

Social Studies, 20.10.2019 20:00

Biology, 20.10.2019 20:00

English, 20.10.2019 20:00

Chemistry, 20.10.2019 20:00

History, 20.10.2019 20:00

World Languages, 20.10.2019 20:00


Health, 20.10.2019 20:00


History, 20.10.2019 20:00

Computers and Technology, 20.10.2019 20:00

Mathematics, 20.10.2019 20:00




 is expected to have a dipole moment of zero.
is expected to have a dipole moment of zero. distance (in cm)
distance (in cm)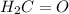 is a polar molecule so it will have dipole moment.
is a polar molecule so it will have dipole moment.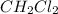 is a polar molecule so it will have dipole moment.
is a polar molecule so it will have dipole moment.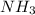 has nitrogen atom as more electronegative than hydrogen atom. So, net dipole moment will be in the direction of nitrogen atom.
has nitrogen atom as more electronegative than hydrogen atom. So, net dipole moment will be in the direction of nitrogen atom.

