
Chemistry, 12.06.2021 18:40 lisamccray45
A gaseous mixture of O2 and N2 contains 35.4 % nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 0.918 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A gaseous mixture of O2 and N2 contains 35.4 % nitrogen by mass. What is the partial pressure of oxy...
Questions

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Social Studies, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

History, 15.09.2020 01:01

History, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

Mathematics, 15.09.2020 01:01

History, 15.09.2020 01:01

English, 15.09.2020 01:01

English, 15.09.2020 01:01

History, 15.09.2020 01:01

English, 15.09.2020 01:01

History, 15.09.2020 01:01

History, 15.09.2020 01:01

History, 15.09.2020 01:01

Biology, 15.09.2020 01:01

English, 15.09.2020 01:01

English, 15.09.2020 01:01

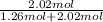 = 0.616
= 0.616

