
Chemistry, 12.06.2021 14:00 shetherealbrat
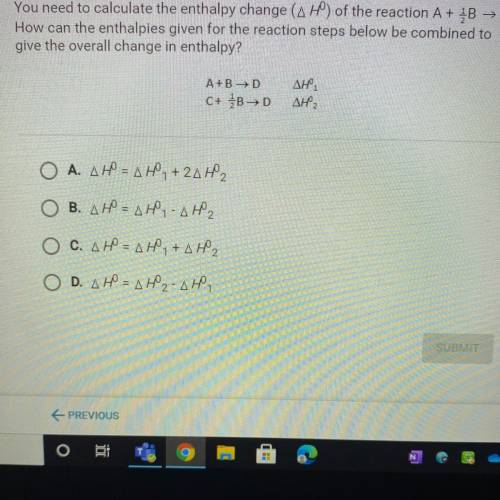
You need to calculate the enthalpy change (AH) of the reaction A + B → C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
A+B →D
C+ B>D
ΔΗ,
ΔΗ,
Ο Α. ΔΗ = ΔΗ, + 2ΔΗ,
B. AH = AH - AH2
O C. AHN AH,+AH2
O D. AH = AH2-AH,

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
You need to calculate the enthalpy change (AH) of the reaction A + B → C.
How can the enthalpies gi...
Questions





Geography, 26.08.2019 07:30

Social Studies, 26.08.2019 07:30




History, 26.08.2019 07:30


Physics, 26.08.2019 07:30

Mathematics, 26.08.2019 07:30

Biology, 26.08.2019 07:30

English, 26.08.2019 07:30



Chemistry, 26.08.2019 07:30




