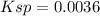Chemistry, 12.06.2021 04:10 morgeron6071
At 20 °C, a saturated aqueous solution of silver acetate, AgCH3CO2, contains 1.0 g of silver compound dissolved in 100.0 mL of solution. What is the Ksp of silver acetate?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
At 20 °C, a saturated aqueous solution of silver acetate, AgCH3CO2, contains 1.0 g of silver compoun...
Questions

Biology, 16.09.2019 10:50

History, 16.09.2019 10:50


Social Studies, 16.09.2019 10:50





Biology, 16.09.2019 10:50

Mathematics, 16.09.2019 10:50



Social Studies, 16.09.2019 10:50


Mathematics, 16.09.2019 10:50



Mathematics, 16.09.2019 10:50

Mathematics, 16.09.2019 10:50

Social Studies, 16.09.2019 10:50

![Ksp=[Ag^+][CH_3COO^-]](/tpl/images/1372/3552/ae7eb.png)
![[Ag^+]=[CH_3COO^-]=\frac{1.0g/(166.9122 g/mol)}{0.1000L} =0.0600M](/tpl/images/1372/3552/f5157.png)