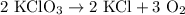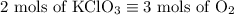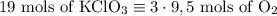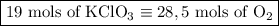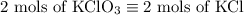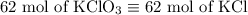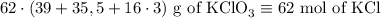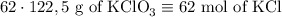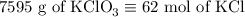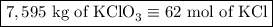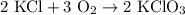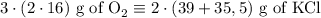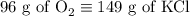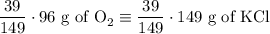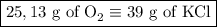2) 2KClO3 --> 2KCl + 3O2
a) How many moles of O2 are produced from 19 moles of KClO3?
b)...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
Questions

Mathematics, 19.11.2020 01:40

Spanish, 19.11.2020 01:40

History, 19.11.2020 01:40




Physics, 19.11.2020 01:40



Mathematics, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40


Mathematics, 19.11.2020 01:40


English, 19.11.2020 01:40

Chemistry, 19.11.2020 01:40

English, 19.11.2020 01:40



Mathematics, 19.11.2020 01:40