Chemistry, 11.06.2021 02:30 karinalovez
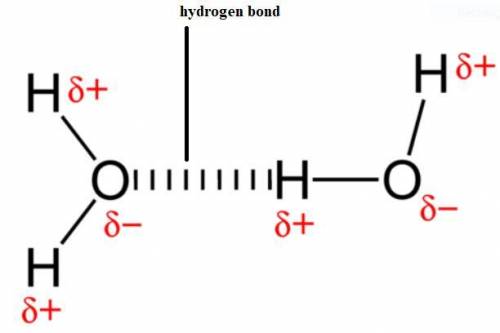
In which of the following molecules is hydrogen bonding likely to be the most significant component of the total intermolecular forces??
a. C6H13NH2
b. CH3OH
c. CH4
d. C5H11OH
e. CO2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

You know the right answer?
In which of the following molecules is hydrogen bonding likely to be the most significant component...
Questions



Chemistry, 13.11.2020 05:00


Social Studies, 13.11.2020 05:00


Mathematics, 13.11.2020 05:00



Mathematics, 13.11.2020 05:00

Mathematics, 13.11.2020 05:00




Mathematics, 13.11.2020 05:00