
Chemistry, 10.06.2021 23:20 jonathon3957
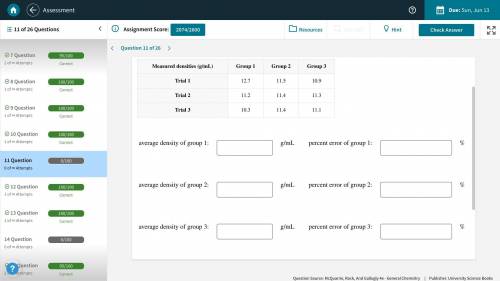
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are attempting to determine the density of a lead weight by various methods. Calculate the average density measured by each group, and the percentage error in each average.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
(Picture Included) A handbook lists the density of lead as 11.3 g/mL. Several groups of students are...
Questions





Physics, 16.09.2019 08:30


Mathematics, 16.09.2019 08:30


Geography, 16.09.2019 08:30

Mathematics, 16.09.2019 08:30

Biology, 16.09.2019 08:30


Biology, 16.09.2019 08:30

Mathematics, 16.09.2019 08:30


Mathematics, 16.09.2019 08:30



Mathematics, 16.09.2019 08:30

Mathematics, 16.09.2019 08:30



