Chemistry, 10.06.2021 23:10 jocelynfray16
Assume that the reaction of aqueous hydrobromic acid solution and potassium hydroxide base undergoes a complete neutralization reaction.
a. Write a balanced chemical equation.
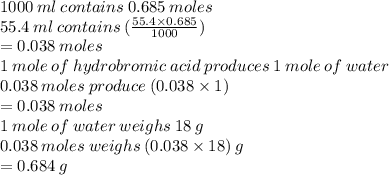
b. How many grams of water can be produce from the complete reaction of excess hydrobromic acid and 55.4 mL of 0.685 M potassium hydroxide solution, assuming that potassium hydroxide is the limiting reactant?
A solution of hydrobromic acid is formed by dissolving 5.00 grams in enough water to make 1.5 L solution.
c.. What was the molarity of this solution?
d. What is the pH of this solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1


Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Assume that the reaction of aqueous hydrobromic acid solution and potassium hydroxide base undergoes...
Questions




Mathematics, 30.03.2020 18:02


Mathematics, 30.03.2020 18:02



Mathematics, 30.03.2020 18:03


Computers and Technology, 30.03.2020 18:03





Health, 30.03.2020 18:03

German, 30.03.2020 18:03