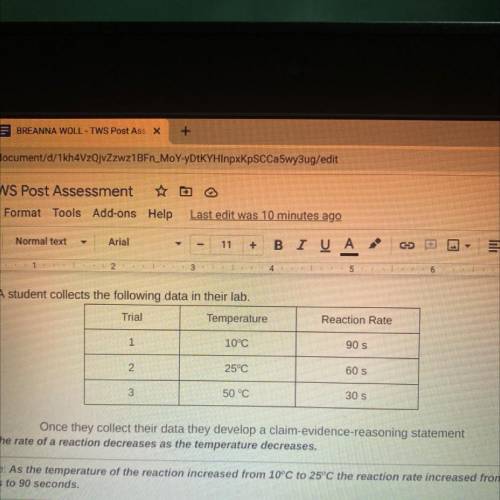
8. A student collects the following data in their lab.
Trial
Temperature
Reaction Rate<...

Chemistry, 10.06.2021 20:30 giusto1073
8. A student collects the following data in their lab.
Trial
Temperature
Reaction Rate
Once they collect their data they develop a claim-evidence-reasoning statement
Claim: The rate of a reaction decreases as the temperature decreases.
Evidence: As the temperature of the reaction increased from 10°C to 25°C the reaction rate increased from 60
seconds to 90 seconds.
Reasoning: Reaction rate depends on the temperature of the reactants. So when the temperature decreases, so
should the reaction rate. Particles need to collide in order to react. Lower temperatures allow molecules to move
slow enough to stick together and react so the reaction rate decreases.
Use the space below to provide feedback to the student. Was their conclusion correct? How should they fix it?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Questions




Computers and Technology, 19.06.2020 17:57



Mathematics, 19.06.2020 17:57

English, 19.06.2020 17:57

Mathematics, 19.06.2020 17:57


Mathematics, 19.06.2020 17:57


Mathematics, 19.06.2020 17:57






Law, 19.06.2020 17:57



