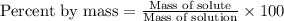Chemistry, 10.06.2021 01:00 caseypearson377
PLSS HELP
Which of the following is the correct formula to calculate percent by mass? (5 points)
O [Mass of solution (g) = mass of solute (9)] x 100
O [Mass of solute (9) = mass of solution (9)] < 100
[Mass of solute (g) - mass of solution (9)] x 100
O [Mass of solution (g) + mass of solute (9)] x 100

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

You know the right answer?
PLSS HELP
Which of the following is the correct formula to calculate percent by mass? (5 points)
Questions

Chemistry, 08.04.2020 20:18


Mathematics, 08.04.2020 20:18

Mathematics, 08.04.2020 20:18

Social Studies, 08.04.2020 20:18


Advanced Placement (AP), 08.04.2020 20:18


Mathematics, 08.04.2020 20:18


History, 08.04.2020 20:18

Mathematics, 08.04.2020 20:18

Mathematics, 08.04.2020 20:18

Mathematics, 08.04.2020 20:18

Biology, 08.04.2020 20:18


Mathematics, 08.04.2020 20:18

Geography, 08.04.2020 20:18

Biology, 08.04.2020 20:18

Advanced Placement (AP), 08.04.2020 20:18